BLC® with Hexvix® allows you to detect bladder cancer missed under White Light Cystoscopy alone1,2,3
Hexvix® is a drug that is taken up by cancer cells in the bladder making them glow bright pink during BLC®.11



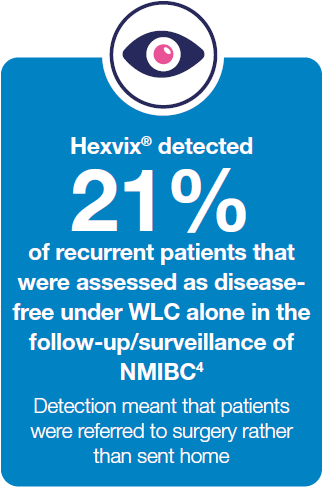
Hexvix® detects bladder cancer missed under White Light Cystoscopy alone3,4,5
Hexvix® can
be used during
surgical treatment
and surveillance
/follow-up

In 25%
of patients at least 1
additional lesion was
found with Hexvix®3
Hexvix® detects patients with CIS that would be missed under WLC alone2
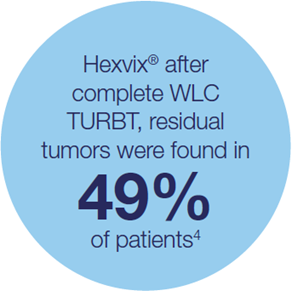
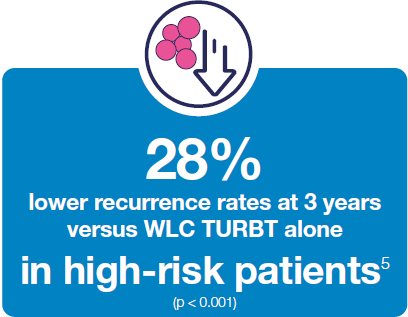
Hexvix® TURBT procedures led to reduced recurrence rates versus WLC TURBT alone 3,5,15
This message is only to be used if specifically requested by the Physician and should not be proactively communicated
- The above is to avoid having Hexvix® niched for use in high-risk patients, when it can benefit intermediate risk patients as well
- Earlier detection will lead to better patient outcomes
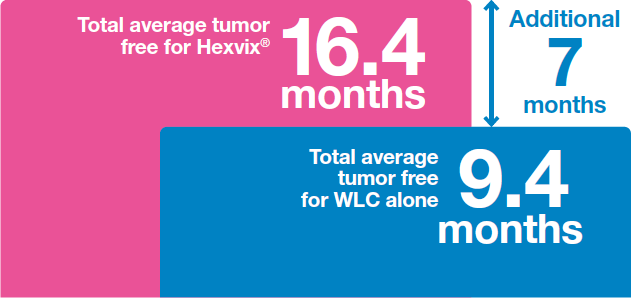
Hexvix® led to increased time to recurrence12
Patients remained on average an additional 7 months tumor free when Hexvix® was used versus WLC alone12
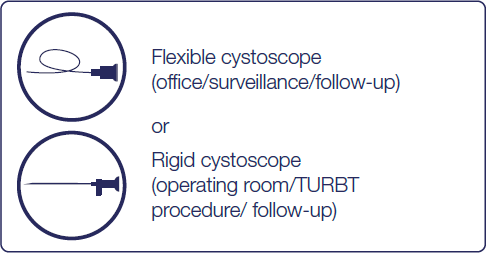
Hexvix® can be used during surgical treatment and surveillance/follow-up
Hexvix® guided TURBT demonstrated to be an independent predictor for improved survival after radical cystectomy19
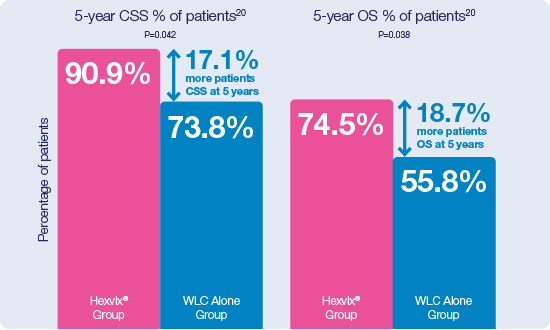
CSS: Cancer Specific Survival; OS: Overall Survival.
Hexvix® should be used for the first TURBT and for all intermediate and high-risk patients, during surgical treatment and surveillance/follow-up7,8,9
How is Hexvix® used
Recommended use cases7,8,9
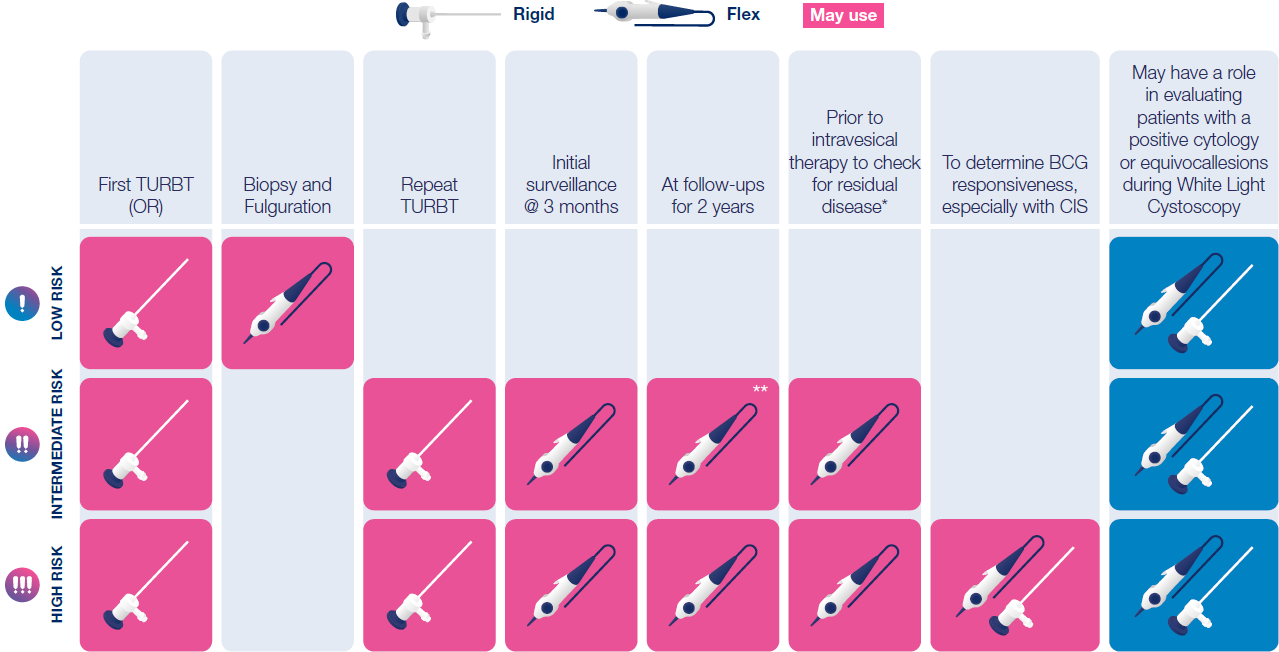
*primarily for referred patients
**consensus on frequency was not reached